All-in-one clinical study management software for your organization
Track Progress
Manage and track core information and workflows during your study life cycles in one platform. Be aware of upcoming study events and milestones by receiving email notifications. Assign tasks to users and keep track of all open tasks.
Learn MoreControl & Collaborate
Invite internal and external team members to join your Castor SMS environment, from anywhere in the world.
Learn More
Secure & Accessible
Store all study documents in one place to ensure they are available and accessible for study staff and other (external) stakeholders. Comply with all security requirements and legislation. Castor has an online manual that is always accessible and a world-class customer success team to provide further assistance.
Learn MoreStay on top of your research projects
A full overview in one place
A full overview in one place
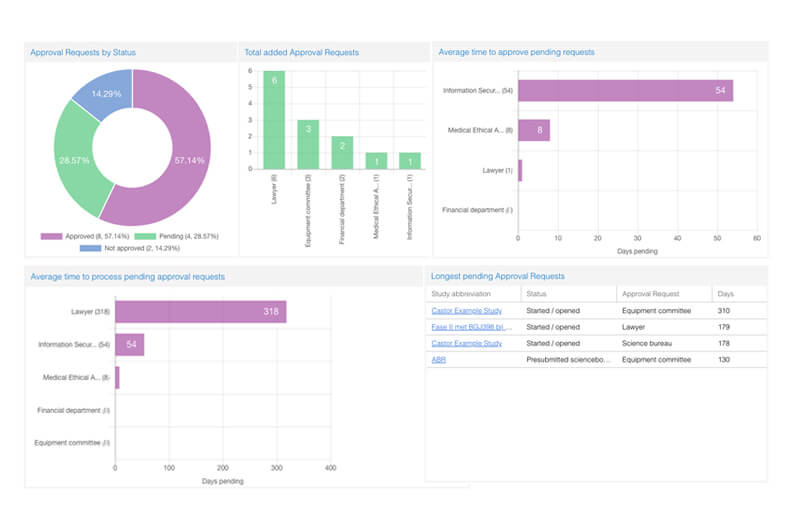
- Gain visibility across research projects at every project stage
- Dashboards for approval requests and study amendments
- Mark your favorite projects and view your last viewed projects
- Track ongoing study amendments
Stay up to date
Stay up to date
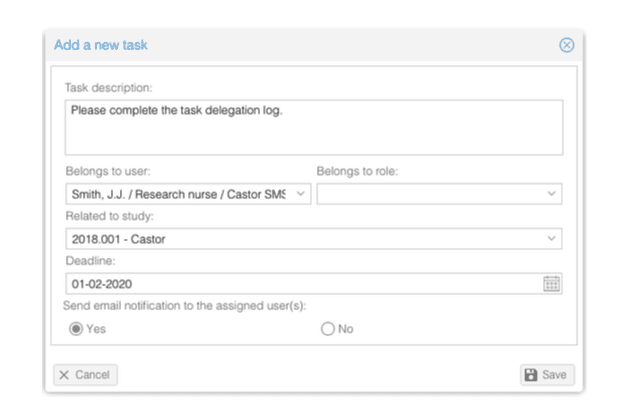
- Set up automatic tasks and email notifications for study events and milestones
- Create tasks and assign them to studies, users or user roles
- Define your own email templates
- Search all projects using study characteristics
- Standardize and track research project information
- Create progress reports
Efficiently collaborate
Designed for collaboration
Designed for collaboration
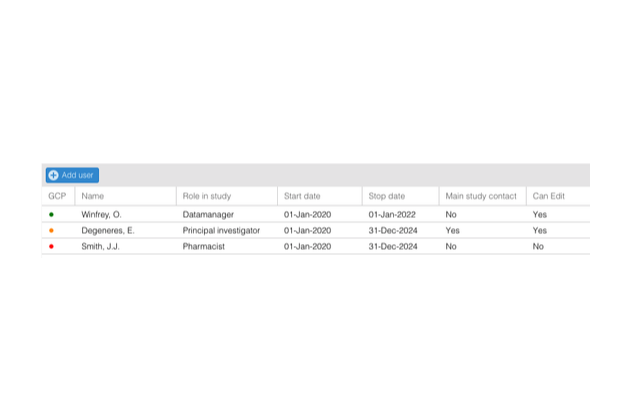
- Add unlimited users
- Activate and deactivate users
- Track team members for each study
- Manage staff documentation, like GCP certificates and CVs
- Manage roles and rights at a user level
- Manage your participating study sites
- Request study approvals and collect electronic signatures
- Invite team members to participate
- Ensure all required study information is available using customizable workflows
- Easily collect approvals for VGO part B
Quick document access
Quick document access
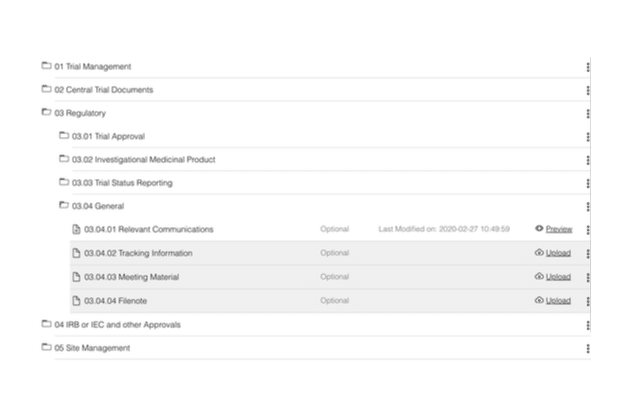
- Define your own folder structure
- Store (upload, download, archive, remove, and hard-delete) documents
- Use bulk actions across multiple documents for efficient document management
- Assign versions and statuses to documents
- Quick view PDF files within the browser
- Populate document templates automatically using data from each research project
Secure your data and get support when needed
Always secure and accessible
Always secure and accessible
- Castor is ISO 27001, 27002, and 9001 certified
- Easily-searchable, 21 CFR Part 11 compliant audit trail of all changes and views
- Strong password requirements
- Easy online access – all standard web browsers supported
- Data servers in Europe, the United Kingdom, and the United States
- Available in English and Dutch
- Export data in Excel format
Support and integrations
Support and integrations
- 24X7 Basic phone support for admin users
- Online public support manual, including video tutorials
- Free email support for all users during working hours
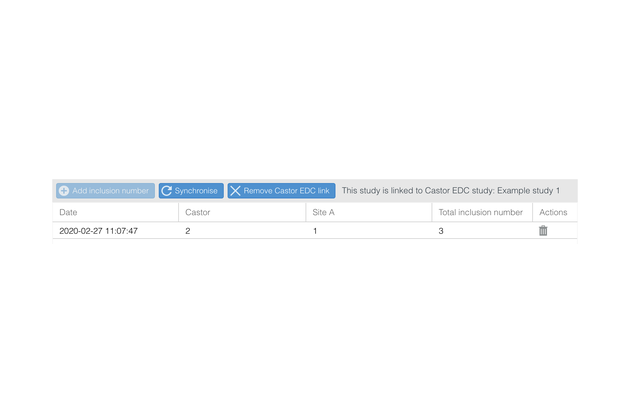
- Integrate with Castor EDC to ensure always-up-to-date inclusion numbers